Kaizen (from the Japanese “kai” for “change” and “zen” for “for the better”) is a management approach to continuous change and improvement, which has led to great success in the lean production of Japanese automobile manufacturers and has been applied in this part of the world since the 1990s as “CIP” (continuous improvement process) in all areas of production and quality management. In the following, we will introduce you to seven management methods in the continuous improvement process (CIP) that could be useful to you:
1. PDCA cycle
The PDCA cycle, also known as the Deming Cycle, is considered the origin of many CIP methods and is still used in numerous areas. It is divided into the four phases Plan, Do, Check and Act. As the word “cycle” already suggests, the central aspect of this approach is that the four phases are repeated again and again, thus creating an iterative process.
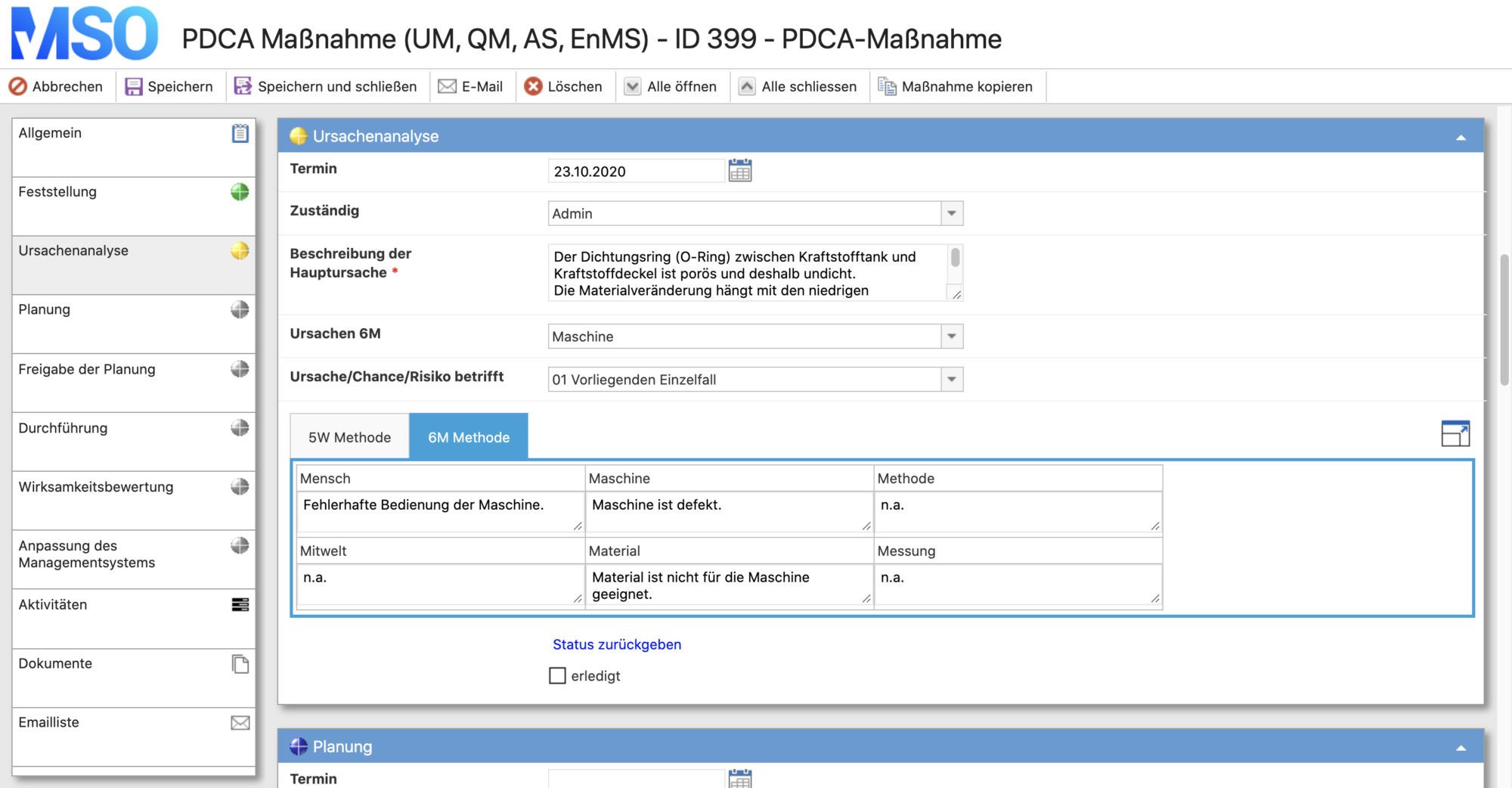
Plan:
- analysis of the actual situation
- target setting
- clarification of the W-questions: who has to do what by when and in which way?
- create a plan
Do:
- implementation of the planned measures
Check:
- checking the results (effectiveness analysis) of the implemented measures
Act:
- depending on the result of the effectiveness analysis, transfer of the measures/recommendations for action into the management system and thus transfer into regular operation
Do you have questions about the implementation with MSO? Then please do not hesitate to contact us.
2. DMAIC cycle (Six Sigma)
The DMAIC cycle is based on the basic considerations of the PDCA cycle and forms the core process of the Six Sigma quality management approach (systematic procedure for process improvement using analytical and statistical methods). The goal is to maintain the performance level of the defined Six Sigma key performance indicators at certain target values using a systematic approach.
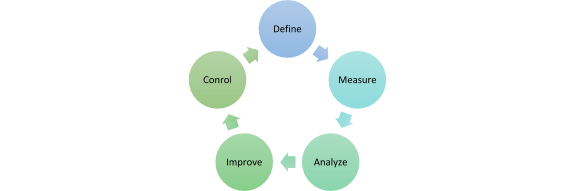
The process is iteratively structured and consists of five recurring phases: Define, Measure, Analyze, Improve and Control.
Define:
- define project goals and responsibilities
Measure:
- development and derivation of measurable parameters
- data preparation and determination of a first Sigma performance level
Analyze:
- identify causes of error
- g. with the help of a failure mode and effect analysis (FMEA)
Improve:
- implementation of identified solutions
Control
- review of the implemented results
- conversion of the implemented solutions into standard processes
Having questions about the implementation with MSO? Then please do not hesitate to contact us.
3. CAPA process
CAPA stands for Corrective and Preventive Action. It is a systematic method of learning from mistakes and not repeating them in the future, which is mainly used in the medical industry. A distinction is made between corrective actions, which are initiated after the occurrence of an error, and preventive actions, which prevent the occurrence of potential deviations.
The following steps are to be followed within the scope of the corrective measures:
- assessment of non-conformities
- determination of the causes of non-conformities
- assessment of the need for action to prevent recurrence
- planning and documentation of necessary measures
- implementation of the measures
- verification that the corrective actions do not affect the ability to meet applicable regulatory requirements or have a significant impact on the safety and performance of the product
- evaluation of the effectiveness of the corrective actions taken
The following steps must be followed as part of the preventive measures:
- identification of potential non-conformities
- identifying the causes of potential non-conformities
- planning and documentation of necessary measures
- implementing the necessary measures
- verification that the measures neither affect the ability to meet applicable regulatory requirements nor have a lasting effect on the safety and performance of the product
- evaluation of the effectiveness of the preventive measures taken
Having questions about the implementation with MSO? Then please do not hesitate to contact us.
4. 8D report (8D method)
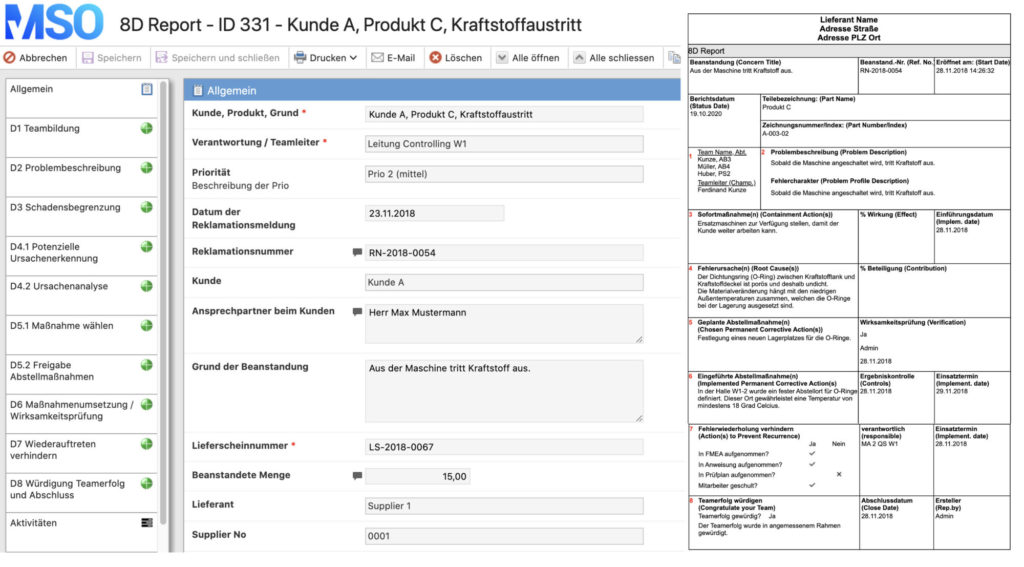
The 8D method includes a systematic complaints management approach with the aim of permanently eliminating problems/errors. In the automotive industry, the 8D report is the central document of complaint management between suppliers and OEM. Based on the PDCA cycle, the 8D method is divided into eight phases:
- form a team
- describe the problem
- take immediate measures
- analyze causes (e.g. Ishikawa diagram or 5-Why method)
- define corrective actions (incl. effectiveness check)
- anchoring corrective measures in the organization
- take preventive measures
- conclude the problem solving process
Having questions about the implementation with MSO? Then please do not hesitate to contact us.
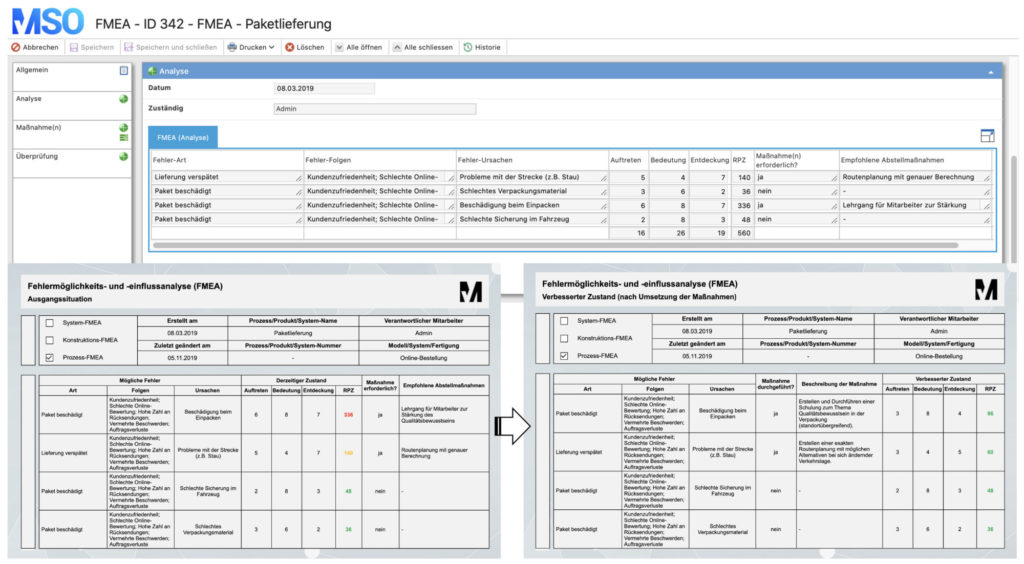
5. Failure Mode and Effects Analysis (FMEA)
FMEA is a method for systematic recording and avoidance of errors. It is often used within the PDCA cycle, the DMAIC cycle, the CAPA process or the 8D method. Depending on the object under consideration, a distinction is made between design, system, hardware, software and process FMEA.
- First, all potential errors are recorded in a list.
- Then the defects are evaluated using a key figure, the so-called risk priority number (RPN). This number is composed as follows:
- Consequences or the significance of an error (S)
- Probability of occurrence (O)
- Probability of detection (D)
- RPN = S*O*D
- If a defined limit value of the risk priority number is exceeded (e.g. RPN > 125), measures must be taken to lower the key figure.
- These measures can serve to minimize the consequences of the failure, reduce the probability of occurrence or increase the probability of detection.
- After the implementation of the defined measures, the RPN is re-evaluated.
Having questions about the implementation with MSO? Then please do not hesitate to contact us.
6. QFD method
Like Kaizen, the Quality Function Deployment (QFD) was developed in Japan in the 1960s and was first used in the automotive industry. Today it is used worldwide in product development, quality and product management. Products, product features, functions or services are systematically analyzed to determine how they meet customer requirements (compared to the competition). The individual elements of the QFD are usually brought together in the form of a matrix or House of Quality.
The following questions apply to the lines: “What does the customer need?, What does he want?, What is needed?, What makes sense for everyone?, What should be achieved?” The questions of the quality characteristics on column level are: “How do you get it?, How do you produce it?, How do you use it?, How should it be achieved?”
The QFD method derives in several steps, which product feature, which function or which performance feature has to be designed, changed and improved how in order to meet the customer requirements and to achieve the greatest success in competition.
- product planning: customer requirements become product performance features
- component planning: performance characteristics become product components, parts or subfunctions
- process and test planning: the product components become process and test characteristics for production and assembly
- production planning: requirements for production and the manufacturing and production technologies used are derived from the process and test characteristics
Having questions about the implementation with MSO? Then please do not hesitate to contact us.
7. 5S cycle
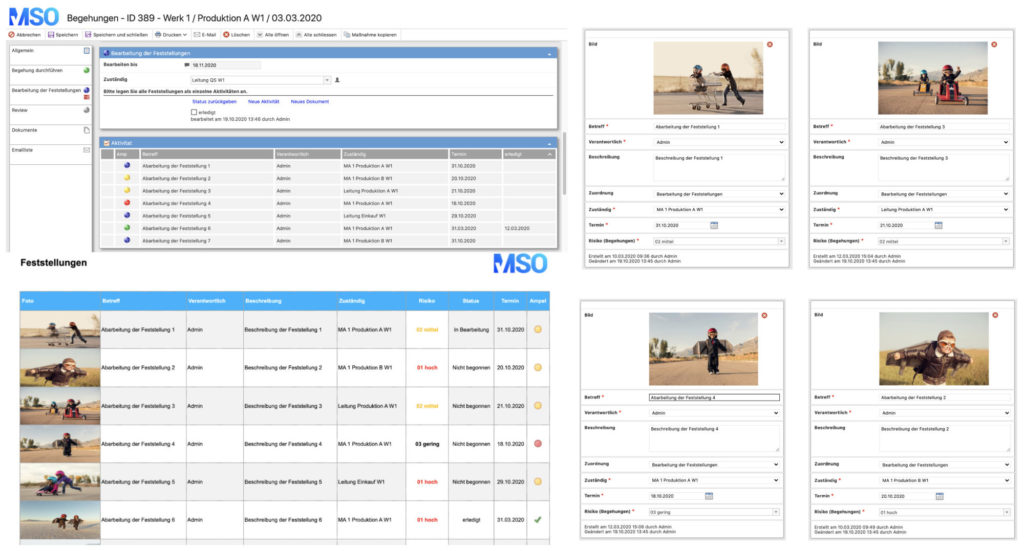
The 5S method also has its origins in the Toyota production system and is the Japanese method for cleanliness, order and safety. In the 5S cycle, the five steps of sorting, setting, shining, standardizing and self-discipline are passed through.
- Sorting:
- in the sense of sorting out
- separation of things that are not needed for the execution of the work
- space for the essential
- Setting:
- development of a system so that everything takes its defined and marked, fixed place
- arrangement according to the frequency and/or sequence of use
- visualization (e.g. shadow tables, images of the target state) to quickly detect deviations from the target state
- Shining:
- the cleaning/purifying process is always an inspection, as deviations and defects can be detected during the cleaning process
- identification of the cause of pollution and sustainable elimination
- Standardizing:
- defining standards within a sector or, if possible, across sectors
- one standard is, e.g., the use of uniform color codes
- cleaning plans define the standard regarding the cleaning process, which items, surfaces or areas are to be cleaned when and how and what is to be paid particular attention to (critical areas in machines or plants)
- the creation of 5S audit forms or checklists is helpful to identify and document deviations from the standard. Systematically identifying deviations in order to subsequently remedy them is an essential prerequisite for the 5th S:
- Self-discipline & continuous improvement:
- adherence to the rules of order and cleanliness
- regular checks and recording of deviations from the standard (e.g. by means of a 5S audit or checklist)
- sustainable elimination of the detected deviations
- running through the 5S cycle at regular intervals with the aim of continuously improving the workplace
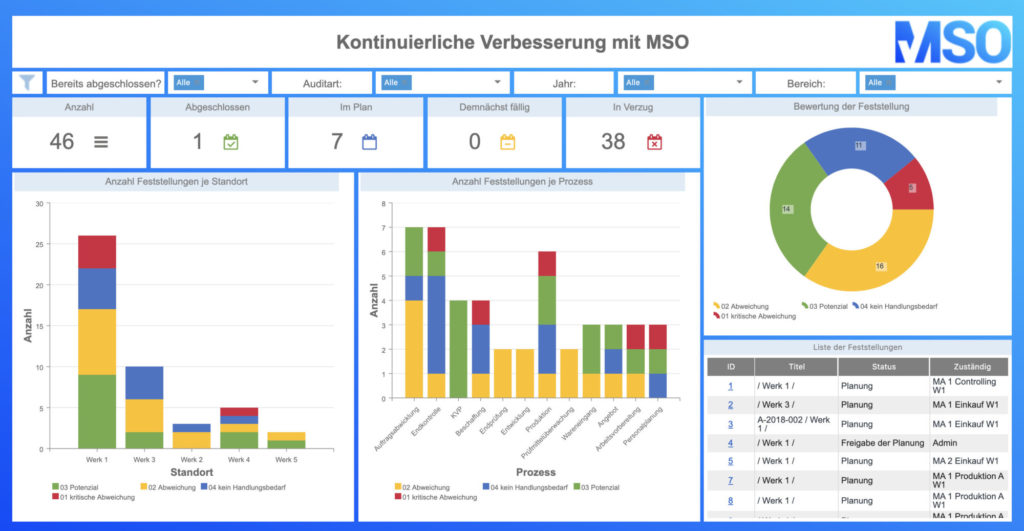
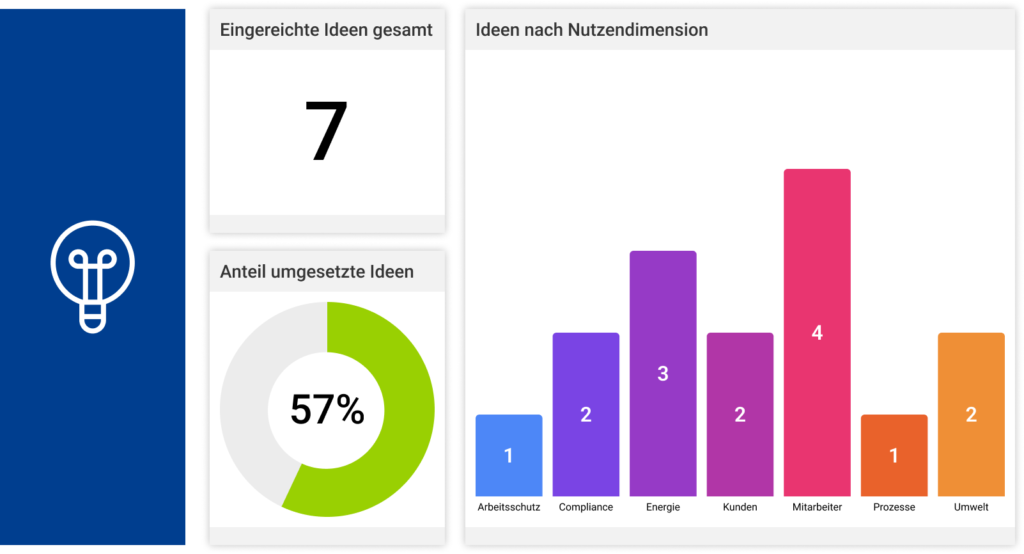
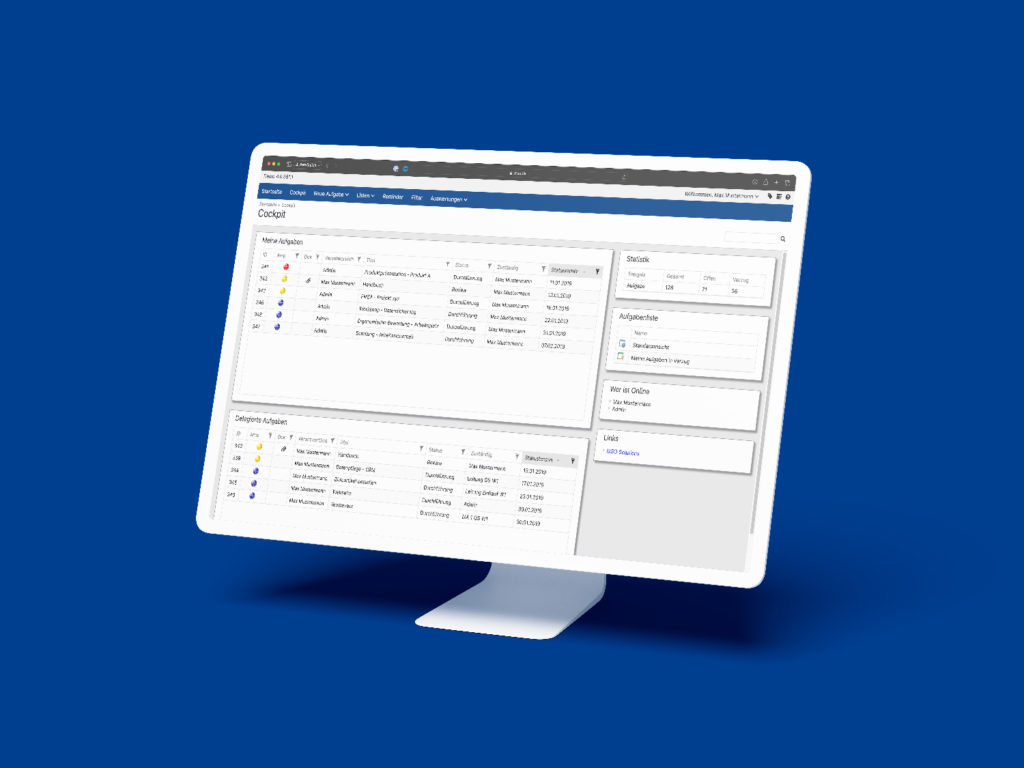
All the CIP methods mentioned above can be sustainably controlled, documented, visualized and implemented with the help of the MSO Action Manager. Do you have any questions about the implementation with MSO? Then please do not hesitate to contact us.